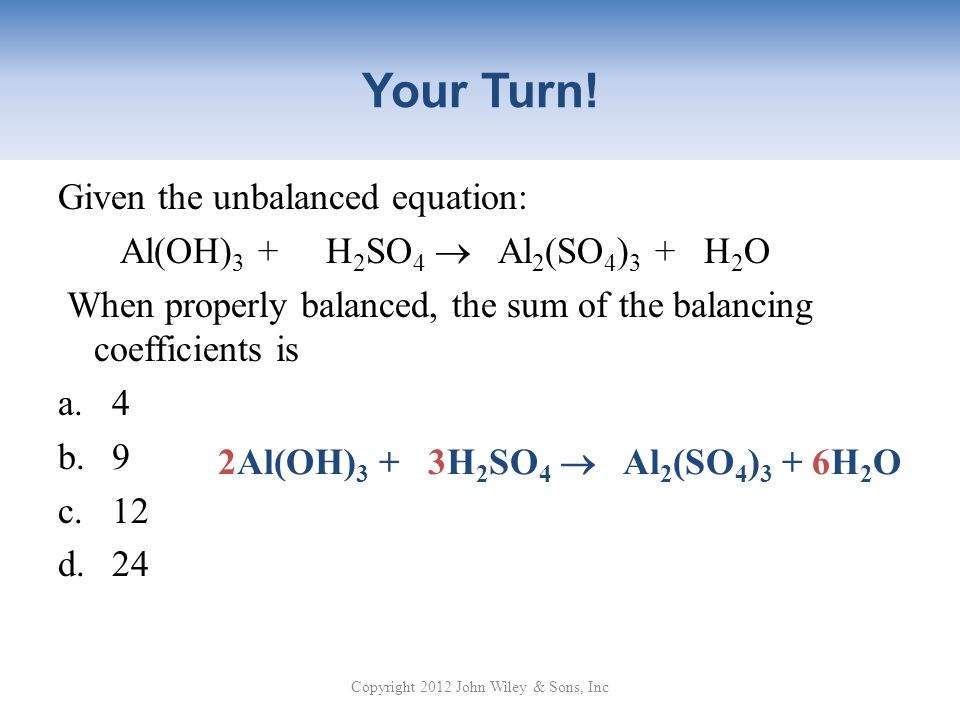
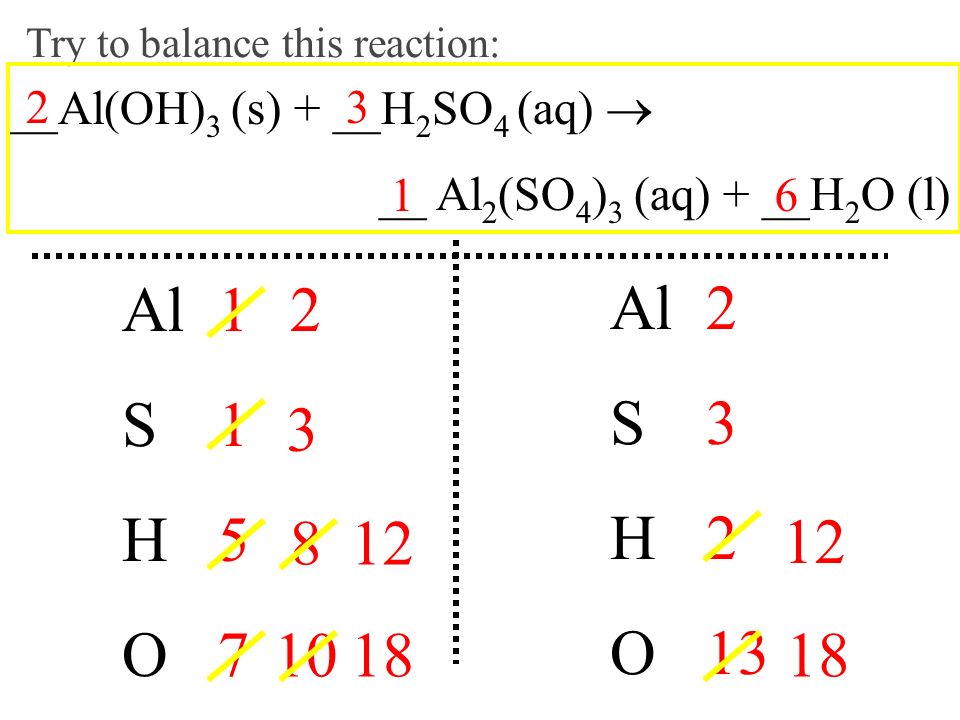
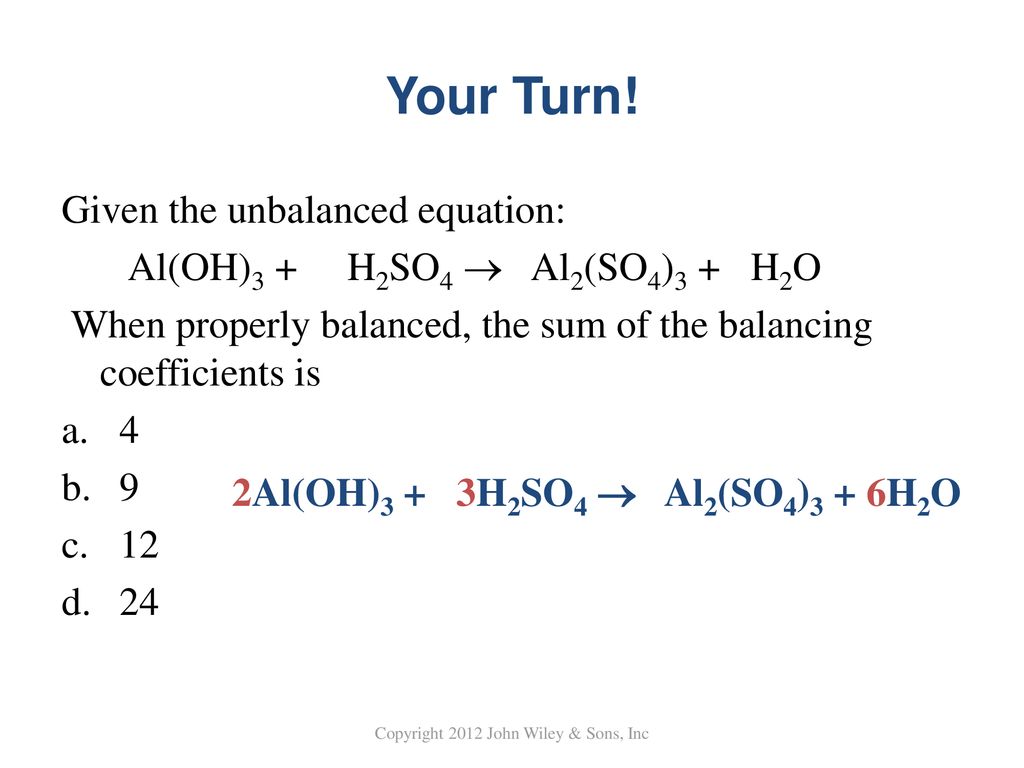
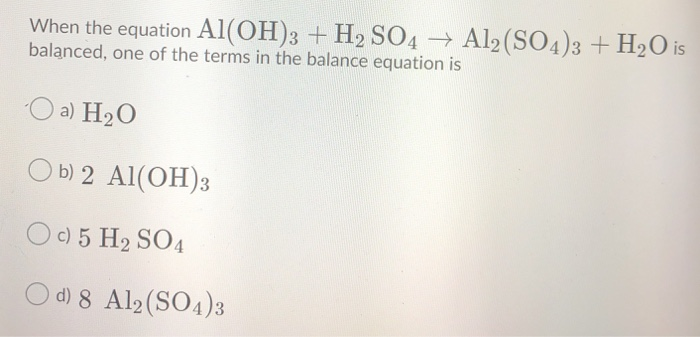H2SO4+Al(OH)3=Al2(SO4)3 +H₂O Balance| Aluminum hydroxide reacts with Sulfuric acid balanced Equation - YouTube

OneClass: Mixing 39.0g of Al(OH)3 with an excess of H2SO4 we produce Al2(SO4 )3. Using the following e...
✓ Solved: When Al(OH)3 reacts with sulfuric acid, the following reaction occurs: 2Al(OH)3+3H2SO4→ Al2(SO4)3+6H2O...

Al(OH)3+H2SO4=Al2(SO4)3+H2O balance the equation @mydocumentary838. al(oh)3+ h2so4=al2(so4)3+h2o - YouTube
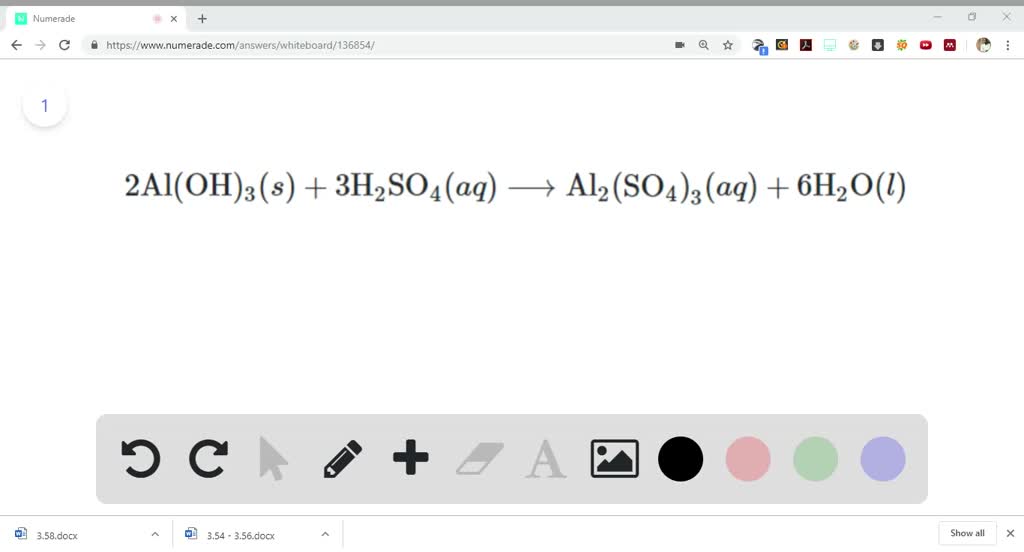
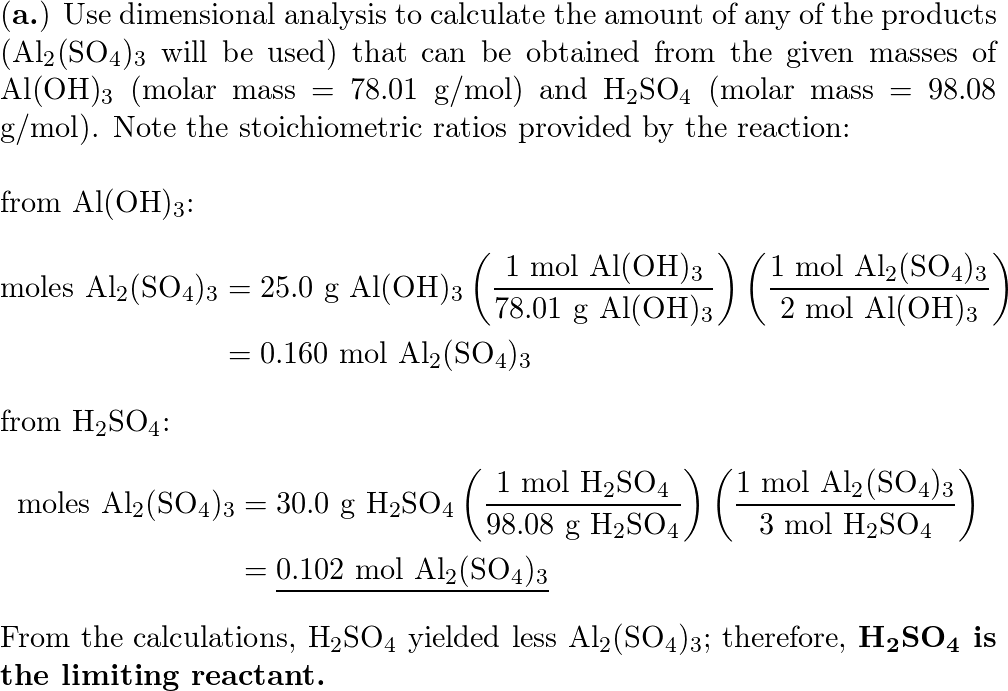
SOLVED:Aluminum hydroxide reacts with sulfuric acid as follows: 2 Al(OH)3 (s)+3 H2 SO4(a q) ⟶Al2(SO4)3(a q)+6 H2 O(l) Which is the limiting reactant when 0.500 mol Al(OH)3 and 0.500 mol H2 SO4 are

SOLVED: Aluminum hydroxide reacts with sulfuric acid as follows: 2 AI(OH)s(s) + 3 H,SO-(aq) Al,(SO4)s(aq) 6 H,O() Which is the limiting reactant when 0.500 mol Al(OH), and 0.500 mol H,SOa are allowed

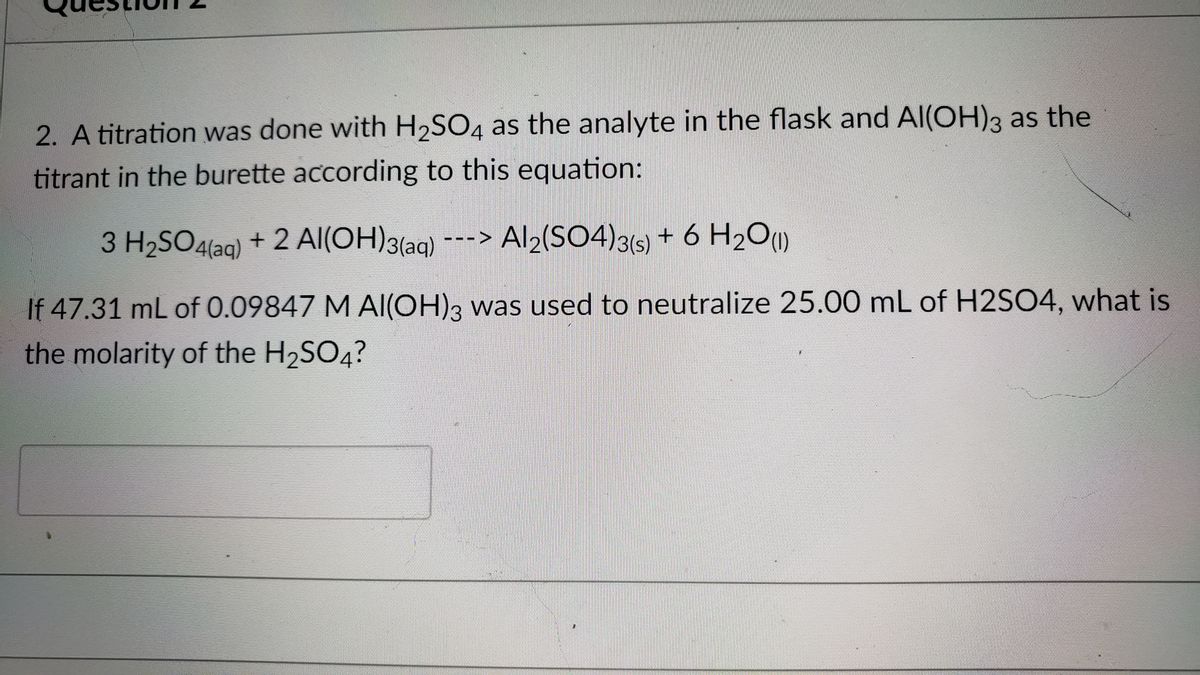
⚗️Based on the equation below, how many moles of aluminum sulfate (Al2(SO4)3) will be produced from - Brainly.com

Alumminum hydroxide reacts with sulfuric acid as follows: 2Al(OH)3+H2SO4--> Al2(SO4)+6H2O. Which reagent is the limiting reactant when 0.500 mol Al(OH)3 and 0.500 mol H2SO4 are allowed to react? How ma | Homework.Study.com